Search the KeriCure product you are looking for right here!
KeriCureTM is committed to developing novel biotechnology-based products to address key issues in skin and wound care. Our HydroShield Technology was developed for over 10 years by the KeriCure scientists, that is the foundation for our skin protectant and liquid bandage product lines currently on the market.
The HydroShield Technology is a unique polymer-based technology that has been designed specifically for topical applications to intact and injured skin. KeriCure maintains multiple pending patents that support this technology and its many applications in the medical industry. Our proprietary polyacrylate polymer is designed to mimic the natural properties of the polymer elastin, which gives the skin its elasticity, for a comfortable, natural barrier when applied. KeriCure products maintain only two simple ingredients, ultrapurified water and a pure proprietary polyacrylate polymer, which allows the natural healing process to occur by providing an effective barrier against dirt and germs.
KeriCure is actively seeking industry partners interested in licensing tailor made formulas based on this technology for areas including medical device coatings, drug delivery, sealants around temporary and permanent semi-implanted devices (catheters, stents, screws, etc.), and others.
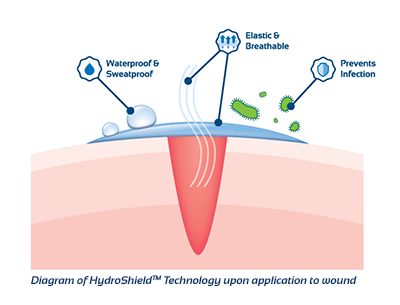
KeriCure HydroShield Benefits
The HydroShield Technology affords KeriCure products that are largely composed of ultrapurified water; much improved over the alcohol, acetone, and petroleum ingredients found in many other liquid bandages. The KeriCure products are formulated with only two ingredients: purified water and a proprietary polyacrylate polymer. This technology provides the KeriCure products with the ability to be chemical and preservative free; these products do not support bacterial or fungal growth and remain “germ free” through multiple applications.
The proprietary polyacrylate polymer is unique to KeriCure products. Optimized specifically for topical application, the polymer was designed to mimic the structure of the all-important protein elastin, which is responsible for the elastic nature of skin and gives skin its ability to bounce back after stretching. The HydroShield polymer exhibits the same mechanical properties as elastin. When applied, the product forms a highly elastic film that can stretch, bend, and be pulled without losing its ability to block germs and provide complete coverage. This ability is only available in the HydroShield Technology, and while many other films are flexible, only KeriCure
products are elastic.
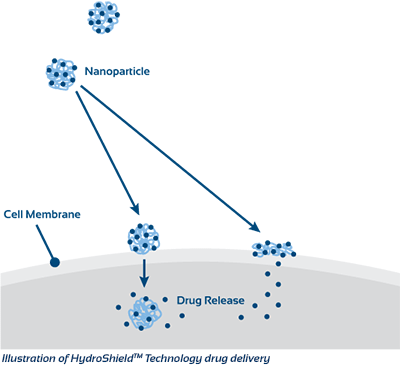
Superior Drug Delivery
The KeriCure technology takes a never before seen approach to drug delivery, by forming a bond between the drug or active ingredient desired and the polyacrylate polymer. This biocompatible system delivers both water-insoluble and water soluble drugs in a unique nanoparticle vehicle, ultimately providing enhanced bioavailability, delivery, and superior controlled release upon application. The polyacrylate polymer is a completely inert, nondegradable polymer, optimal for coatings, permanent implants, and topical application.
Our studies have shown that this ground breaking technology allows antibiotics, such as penicillin, to regain activity against resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA). Through the binding of the active ingredient to the nanoparticle, the drug is able to circumvent resistance measures of the target, in this case MRSA, allowing the nanoparticle to act as a Trojan Horse for the drug. Once actively transported into the cell, the drug or active is released through enzymatic activity and capable of its normal function.
KeriCure is interested in partnering with companies who have theoretical or established drugs
and additives with solubility issues. Our focus is to provide a novel delivery system to our
customers that will afford their product enhanced topical presence and effectiveness through
longevity on the skin and superior drug release. Please contact us at info@kericure.com
if you are interested in partnering or looking for a new and innovative delivery system
to add to your repertoire.
Partnership Opportunities
Our long-term objective in growing the KeriCure brand to the point of national recognition as the leaders in the liquid bandage market is licensing our proprietary product line to a larger, multi-faceted operation. In doing so, we’ll make available the resources for our products’ scalability on an international level. We recognize the limitations to high-volume distribution on an international scale and as such, welcome the opportunity for providing global accessibility to our product through a licensing strategy.

